Abstract
Introduction:
Diffuse large B-cell Lymphoma (DLBCL) is the most common lymphoid cancer in the United States with 30K diagnoses each year. The standard therapy for all newly diagnosed patients is rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (RCHOP) which cures ~60% of patients. Lenalidomide (L) and obinutuzumab (O) have both shown promising efficacy and acceptable toxicity in DLBCL patients. Obinutuzumab, a humanized CD20 monoclonal antibody with increased pre-clinical affinity to the FcGRIIIa receptor in comparison to rituximab, may have increased antibody dependent cell-mediated cytotoxicity (ADCC). Lenalidomide, a potent immunomodulatory, is toxic to DLBCL via interferon signaling stimulation. As single agents, O and L achieve overall response rates (ORR) of 32% and 28%, respectively, in patients with relapsed DLBCL (Morschhauser, 2013, Hernandez-Ilizaliturri, 2011). The addition of L to RCHOP may overcome adverse outcomes of the non-germinal center (non-GCB) subtype of DLBCL. Randomized phase III clinical trials evaluating RCHOP +/- Len in non-GCB DLBCL are highly anticipated. The GOYA study found RCHOP was as effective as OCHOP in patients with unselected newly diagnosed DLBCL. We hypothesize the combination of OL-CHOP will result in enhanced NK cell-mediated cytotoxicity, down-regulation of interferon regulatory factor 4, and a complete response (CR) rate ≥ to OCHOP (57% in GOYA trial, Vitolo et al, ASH 2016).
Methods: Newly diagnosed, CD20+ DLBCL patients were eligible if they have measurable disease, age ≥18 years, and adequate organ function. In the Phase Ib trial, Obinutuzumab was dosed at 1000mg IV days (d) 1, 8, and 15 during cycle 1, and d 1 on cycles 2-6. Lenalidomide dose was 15mg d 1 - 14 cycles 1 - 6 in the first cohort, with lower dose cohorts if toxicities occurred, using a 3+3 design. CHOP was dosed in standard fashion. Once the maximum tolerated dose (MTD) was identified, phase II was initiated with a planned total of at least 50 evaluable patients at the MTD. The primary objectives of this phase Ib/II trial were to determine the MTD and efficacy of LOCHOP. Correlative studies, including minimal disease assessment, cell of origin determination, and immune profiling are planned on blood and tumor samples.
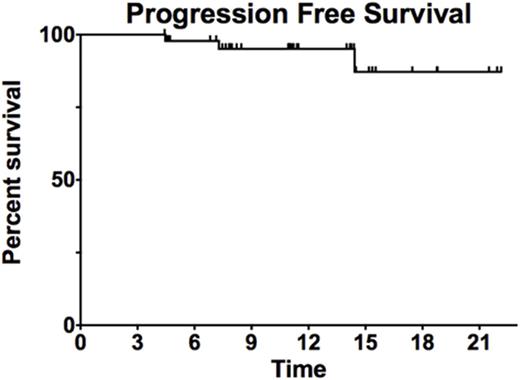
Results: Fifty three patients were enrolled, 6 in Phase Ib trial, 47 in Phase II with all evaluable for safety and 51 for efficacy. Two patients withdrew consent after 1 cycle of therapy. The median age was 62 years (26-83), with 42% males and a median IPI of 2 and NCCN IPI of 3. Five patients had double hit DLBCL, 5 patients had Double Expressor DLBCL, and 21 patients were non-GCB DLBCL. No dose limiting toxicities were identified in in Phase Ib. Toxicities encountered in PhIb include neutropenia (Grade 3: 50%, Grade 4 33%), thrombocytopenia (grade 3: 17%), and rash (grade 2: 17%), with similar findings in PhII. All six patients in PhIb achieved a complete response (CR). In the Phase II trial, 50 patients have end of therapy response assessment as of 8/2/17, with 45 with CR, 4 with partial response, 1 with progression. In the combined evaluable patients, the overall response rate is 98%, with CR of 90%. The CR rate was 90% in non-GCB and 92% in GCB. The median follow up 11 months and the median progression free and overall survival are not reached. There have been no deaths. Additional follow up and analyses will be presented at the meeting.
Conclusions: The combination of Obinutuzumab, Lenalidomide, and CHOP is well tolerated, with no dose limiting toxicity encountered in the phase Ib trial, and efficacy is impressive in this single center single arm Phase Ib/II trial. Adverse events do not appear to be different than expected with standard RCHOP. Final results and correlative analyses will be presented at the ASH meeting
Westin: Apotex: Membership on an entity's Board of Directors or advisory committees; Novartis Pharmaceuticals Corporation: Membership on an entity's Board of Directors or advisory committees; Kite Pharma: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees. Nastoupil: TG Therapeutics: Honoraria, Research Funding; Gilead: Honoraria; Abbvie: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Genentech: Honoraria, Research Funding; Karus Therapeutics: Research Funding; Celgene: Honoraria, Research Funding. Neelapu: Merck: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Poseida Therapeutics, Inc: Research Funding; Cellectis Inc.: Research Funding; Kite Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol-Myers Squibb: Research Funding; Karus: Research Funding; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Lee: KITE PHARMA: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal